Research & Development
RJS’s aim is to develop a simple, easy-to-use and relatively cheap test based on a biomarker found in one of the body fluids especially in saliva. Ideally, the test could be applied within minutes of the initial insult.
The Cancer diagnostic quick test RJS is developing will be proprietary biomarker (s) technology from a non-invasive sample of body fluid approved by regulatory agencies for early diagnosis, screening or prognosis of cancer. Compared to the main state-of-the-art approach, i.e. imaging tests, other tests the RJS kit provides a reliable result rapidly and cost effectively and does not require medical professionals to interpret the result.
Risk factors triggers the activation of a specific oncogenic factor and related-signalling pathway leading to changes biomarkers (our proprietary technology). During pathogenesis of cancer, overexpression of any protein can be cleaved from cancer cells surface and end up directly into saliva. Saliva and blood are two different biofluids are associated to each other on the molecular level and molecules from blood also reach into saliva through various mechanisms such a ultrafiltration and transdiffusion. Integration of RJS proprietary biomarkers technology on RJS proprietary lateral flow technology ( assay) is providing diagnosis, detection, screening and monitoring of precancerous and cancerous in human by using saliva samples.KEY BENEFITS OF THE RJS TEST:
- Quick: RJS provides a definitive result shortly after saliva samples, without the need of specialized labs and the related
waiting time. - Reliable: Based on our pre-clinical trials, the reliability is very high. The increased accuracy can help diagnose.
- Inexpensive: Since no trained staff or laboratory equipment is needed, unlike in other cancer detection methods, the tests are extremely low cost.
- Reduces unnecessary expensive CT/MRI scans: The current gold standard for the diagnosis of cancer are imaging techniques that are expensive. Plus, most hospitals only have one or very few of those testing machines.
- Easy to interpret: the results are as simple to interpret as in a pregnancy test. Positive stands for the presence of cancer and precancerous.
- Get the “better outcome” from the epidemic: Ideally, RJS test can be used in routine checks at the general practitioners to get better outcome.
PROOF OF CONCEPT STUDIES IN HUMANS
CLINICAL STUDY 1
The first clinical study demonstrated that the new biomarker can be used for clinical detection of reflex. Samples from 25 patients and 20 healthy volunteers were collected and those were analyzed in state-of-the-art laboratories to evaluate the presence of the biomarker in the body fluids. The level of biomarker was significantly elevated in patients compared to healthy controls. The result was a significant first milestone for RJS.
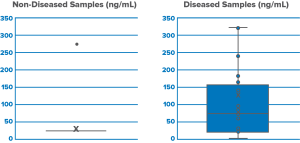
CLINICAL STUDY 2
The first clinical study demonstrated that the new biomarker can be used
for clinical detection of reflex. Samples from 25 patients and 20 healthy volunteers were collected and those were analyzed by using our RJS proprietary biomarkers lateral flow technology to evaluate the presence of the biomarker in the body fluids. The level of biomarker was significantly elevated in patients compared to healthy controls. Also, we are not succeful integration of biomarkers on lateral flow but also detection of biomarkers in saliva samples The result was a significant second milestone for RJS.
